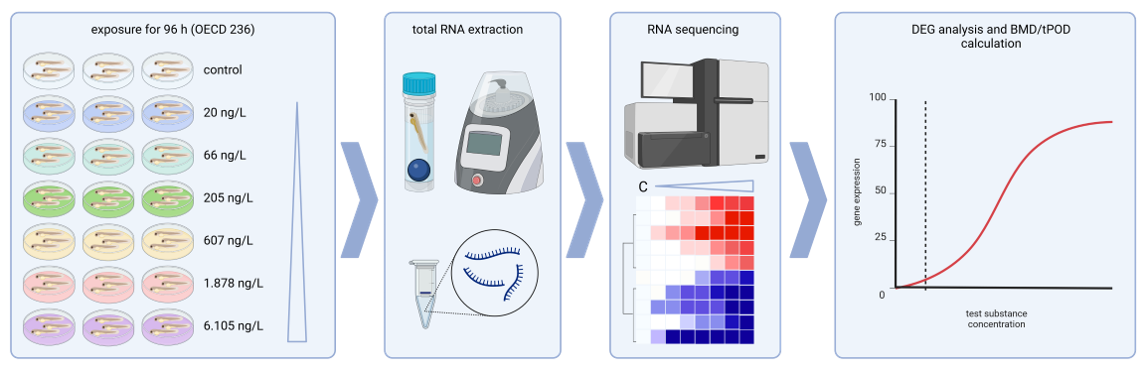
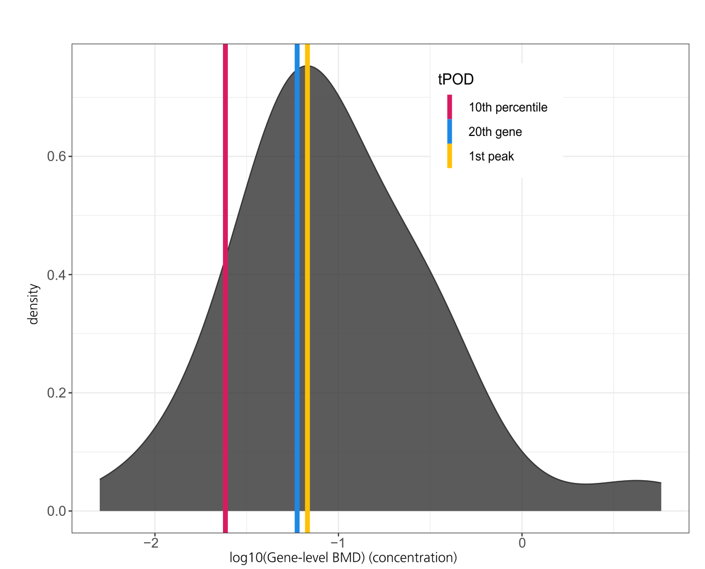
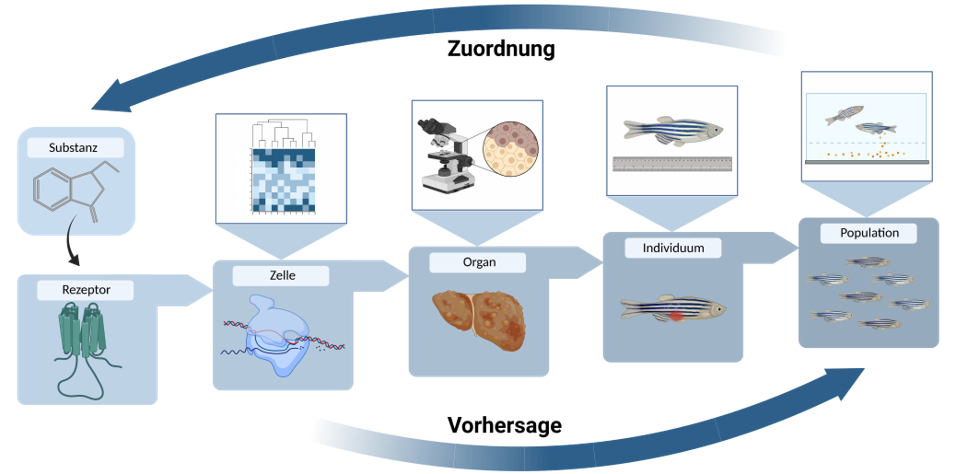
The Session was initiated by ECHA to join views from regulation, industry and academia on further research needs. The chairs started with initial presentations, followed by statements from invited panellists and their answers to a list of questions.
Wim de Coen, ECHA, presented ECHA’s Scientific Research Needs: 1) Provide protection against most harmful chemicals with a focus on immunotoxicity, neurotoxicity and endocrine disruption. This comprises the Identification of the critical windows for the development of the respective system, foundational research on AOPs & establishment of their interlink with existing and potential New Approach Methods (NAMs). Also, thorough identification of reference chemicals to support NAM reliability testing and validation, addressing data gaps regarding toxicological information on metabolites and considering NAMs based on invertebrates. 2) Addressing chemical pollution in the environment. This includes bioaccumulation issues such as the development of non-vertebrate methods to predict the bioaccumulation potential of surfactants, ionisable substances, organometals as well as of super hydrophobic substances. Also, an improved bioaccumulation assessment for air-breathing organisms and for secondary poisoning via the environment especially for mixtures. Furthermore, approaches based on monitoring field data enabling persistence, long-range environmental transport and/or bioaccumulation assessment should be developed and the protection of biodiversity by use of NAMs should be expanded. 3) Shift away from Animal Testing. This comprises the development of case studies for Read across and NAMS, of in vitro/in silico ADME and Physiologically-Based Kinetic models, as well as the validation of a systematic in vitro/silico battery to direct the generation of chronic toxicity data for vertebrate species. 4) Improved availability of chemical data. This includes knowledge and methodologies to support hazard assessment for polymers, including bioavailability, stability to degradation under environmental conditions, bioavailability and relevance for human health hazard assessment. For micro- and nano-sized materials investigations of the long-range transport, uptake and toxicity for humans and other organisms. should be improved. Finally, analytical methods for enforcement, such as the characterisation of nanomaterials, including advance materials should be improved. In his conclusion, he asked for an ongoing dynamic dialogue between all stakeholders.
 Fraunhofer-Institut für Molekularbiologie und Angewandte Oekologie IME
Fraunhofer-Institut für Molekularbiologie und Angewandte Oekologie IME