Plant pests and pathogens are a major threat to crop production worldwide. This has increased dramatically in recent years. Globalization, trade and climate change all play a role. The FAO estimates that between 20 and 40 percent of global crop production is lost to pests each year. Plant diseases, for example, cost the global economy about $220 billion each year. The damage is caused by pathogens such as bacteria, fungi, insects and viruses.
Traditional methods for identifying plant pathogens include observing symptoms and culturing the pathogens for morphological identification under a microscope. Today, widely used diagnostic methods are based on immunological technologies such as enzyme-linked immunosorbent assay (ELISA) or lateral flow immunoassay (LFIA). Nucleic acid-based methods for pathogen detection and identification are gaining acceptance in laboratories. The polymerase chain reaction (PCR) and its variants are usually very specific and sensitive, as well as relatively fast and inexpensive, but they have some disadvantages because they require specialized equipment and trained personnel.
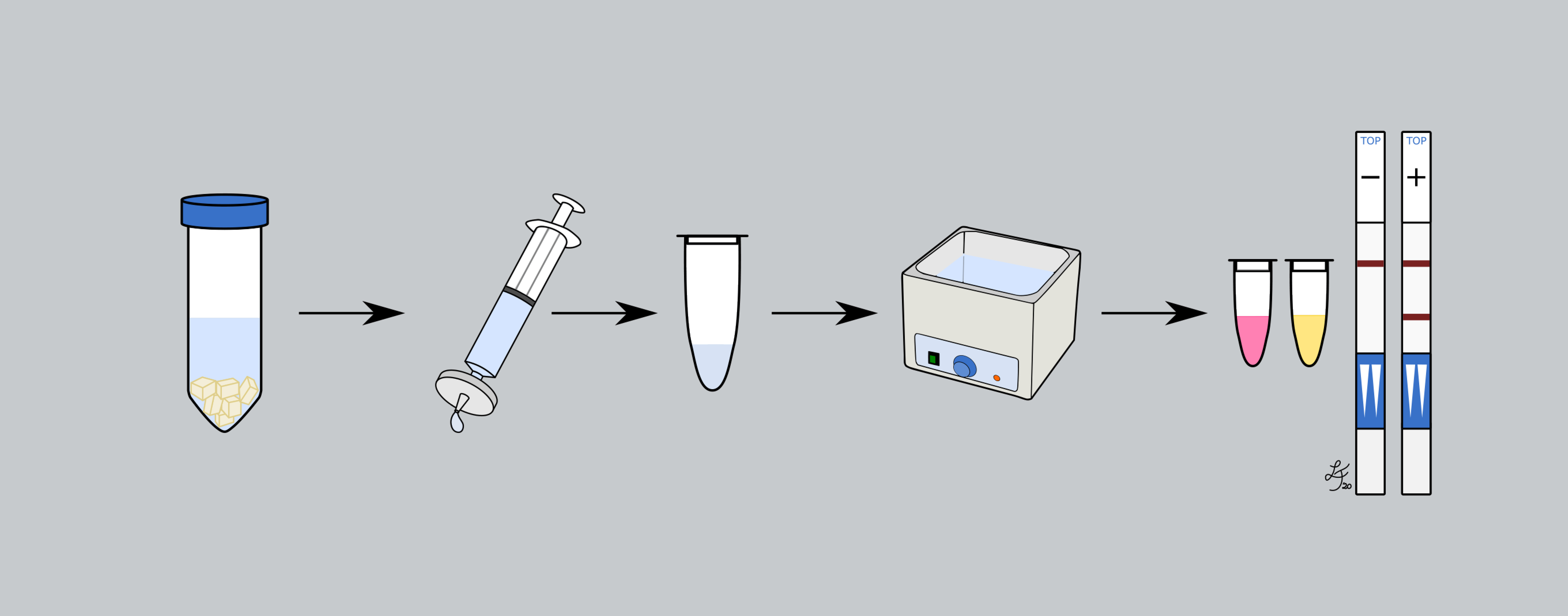
However, to control and, if possible, prevent plant diseases and the spread of pathogens to new areas, it is imperative to develop methods for early detection that can be used in the field. The realization of such point-of-care diagnostics requires the interplay of the following characteristics High specificity, sensitivity, reproducibility, speed, cost-effectiveness and multiplex detection capability combined with ease of use so that tests can be performed by untrained personnel without the need for specialized laboratory equipment.
Technologies based on isothermal amplification of nucleic acids, such as loop-mediated isothermal amplification (LAMP), best meet these requirements for point-of-care diagnostics. Therefore, the Department of Functional and Applied Genomics is focusing its research and development efforts on this technology for PoC diagnostics of plant pathogens.
 Fraunhofer Institute for Molecular Biology and Applied Ecology IME
Fraunhofer Institute for Molecular Biology and Applied Ecology IME