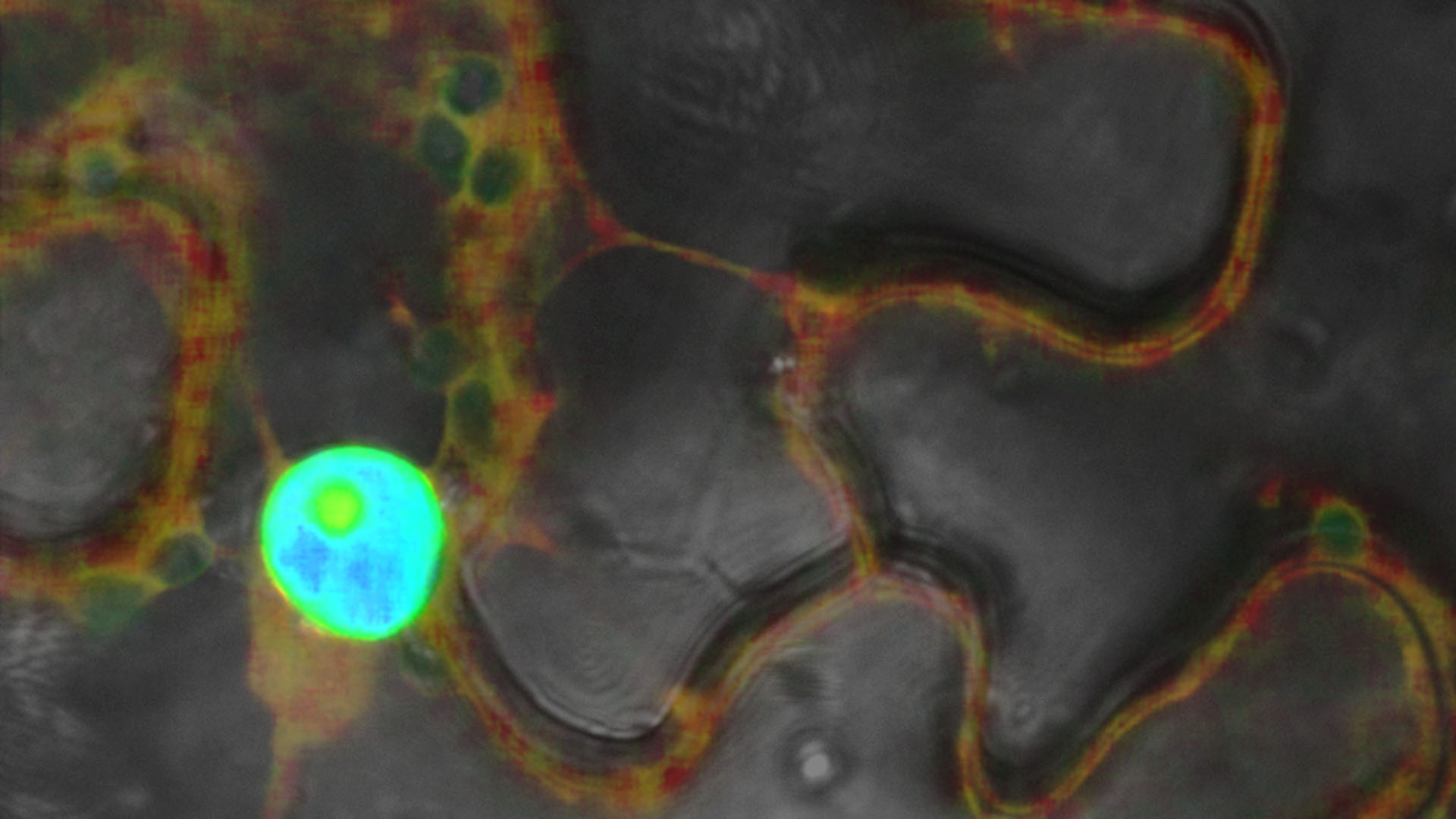
Confocal laser scanning microscopy produces high-resolution, three-dimensional images of biological samples. Unlike light and fluorescence microscopy, this method selectively illuminates the specimen at a specific wavelength using lasers. A scanner with highly sensitive, latest -generation detectors scans the area of interest point by point. Depending on the experimental setup, software compiles the information into a two-dimensional image, three-dimensional structure, or animated image series using a time series. Optical segmentation reduces scattered light and increases contrast, enabling detailed analyses of subcellular structures.
We use the Stellaris 8 FALCON system to image the dynamics of living organisms. By employing a pulsed white light laser and time-correlated single photon counting (TCSPC), additional information about the fluorescence lifetime can be generated in addition to spectrally separated images. A fluorescent dye has a characteristic emission spectrum and a fluorescence lifetime that reflects how long the dye remains in an excited state. This allows for the simultaneous investigation of a large number of fluorescent labels (fluorophore multiplexing) and the analysis of various fluorescent components in living cells. The integrated system for recording fluorescence intensity and lifetime can analyze fast, complex, and dynamic processes, such as biosensor applications and molecular interactions, at high speed.
Additionally, our FRAP (fluorescence recovery after photobleaching) and FRAP-XT studies with an ultra-fast scanner allow us to examine molecular mobility and diffusion kinetics.
Our equipment includes
- DMi8 CS inverted research microscope with motorized sample stage
- White light laser 440-790 nm, pulsed (up to 80 MHz)
- Lasers: Diode 405 nm, Diode 448 nm, OPSL 488 nm, OPSL 514 nm, DPSS 561 nm
- Tandem scanner 8 kHz
- Detectors: 2x HyD-X, 2x HyD-S, 1x HyD-R
- FALCON (FAst Lifetime CONtrast) for fast fluorescence lifetime imaging
- LIGHTNING for adaptive deconvolution up to a lateral resolution of 120 nm
- Hamamatsu ORCA-Flash 4.0 CMOS camera
 Fraunhofer Institute for Molecular Biology and Applied Ecology IME
Fraunhofer Institute for Molecular Biology and Applied Ecology IME