Alternative Testing Methods

Approaches to reduce animal testing
Acute and long-term toxicity tests with fish required for the environmental risk assessment – besides statutory tests on rodents for product safety within the human health risk assessment – contribute to the still rising number of animal tests. However, the development of additional regulatory tests to safeguard environmental and consumer protection are underway. The addition of farmed fish species (rainbow trout, carp) to the spectrum of animals used for food production, which are to be tested in metabolism studies, is currently implemented. Accordingly, the demand for animal test replacement and refinement methods is high, whereas the availability of effective and accepted alternatives is limited. For this reason, the Fraunhofer IME actively engages in the development of fish embryo-based and cellular as well as invertebrate alternative methods.
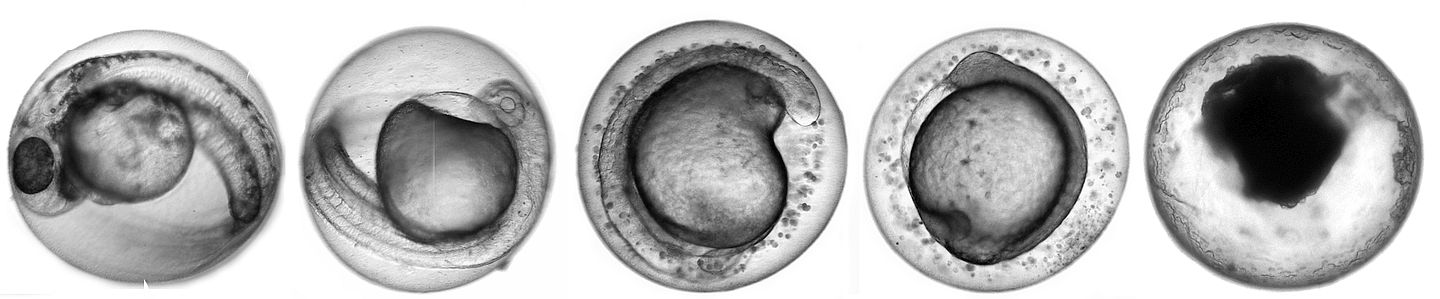
The fish embryo toxicity test (FET) according to DIN 38415-T6, also known as the fish egg test, already replaces mandatory tests on fish for wastewater effluent in Germany since 2005. Internationally, it is being discussed whether to use the FET as a replacement or refinement method also for acute fish tests as required for the registration and evaluation of chemicals. The FET complies with the 3R principles since according to the current European legislation (Directive 2010/63/EC), fish embryos are not considered protected animal.
An ongoing project cooperation with the University of Bern investigates whether fish cell culture studies in combination with kinetic modeling could replace fish bioaccumulation studies (concentration and magnification).
By improving mechanism-specific quantitative structure-activity relationship (QSAR) methodologies the number of animal test required is further condensed.
Selected Publications
Hering I, Eilebrecht E, Parnham MJ, Günday-Türeli N, Türeli AE, Weiler M, Schäfers C, Fenske M, Wacker MG:
Evaluation of potential environmental toxicity of polymeric nanomaterials and surfactants. Environmental Toxicology and Pharmacology (2020) 76:103353
(DOI: 10.1016/j.etap.2020.103353)
Delov, V., Muth-Köhne, E., Schäfers, C., Fenske, M.:
Transgenic fluorescent zebrafish Tg(fli1:EGFP)y1 for the identification of vasotoxicity within the zFET. Aquatic Toxicology 150 (2014) 189–200 (DOI: 10.1016/j.aquatox.2014.03.010)
Schiller, V., Zhang, X., Hecker, M., Schäfers, C., Fischer, R., Fenske, M.:
Species-specific considerations in using the fish embryo test as an alternative to identify endocrine disruption. Aquatic Toxicology 155 (2014) 62–72 (DOI: 10.1016/j.aquatox.2014.06.005)
Muth-Köhne, E., Wichmann, A., Delov, V., Fenske, M.:
The classification of motor neuron defects in the zebrafish embryo toxicity test (ZFET) as an animal alternative approach to assess developmental neurotoxicity. Neurotoxicology and Teratology 34 (2012) No. 4: 413 - 424
(DOI: 10.1016/j.ntt.2012.04.006)
Schiller, V., Wichmann, A., Kriehuber, R., Schäfers, C., Fischer, R., Fenske, M.:
Transcriptome alterations in zebrafish embryos after exposure to environmental estrogens and anti-androgens can reveal endocrine disruption. Reproductive Toxicology 42 (2013) 210-223 (DOI: 10.1016/j.reprotox.2013.09.003)
Schiller, V., Wichmann, A., Kriehuber, R., Muth-Köhne, E., Giesy, J. P., Hecker, M., Fenske, M.:
Studying the effects of genistein on gene expression of fish embryos as an alternative testing approach for endocrine disruption. Comparative Biochemistry and Physiology. Part C: Toxicology & Pharmacology 157 (2013) No. 1: 41-53 (DOI: 10.1016/j.cbpc.2012.09.005)
Turner, C., Sawle, A., Fenske, M., Cossins, A.:
Implications of the solvent vehicles dimethylformamide and dimethylsulfoxide for establishing transcriptomic endpoints in the zebrafish embryo toxicity test. Environmental Toxicology and Chemistry 31 (2012) No. 3: 593-604 (DOI: 10.1002/etc.1718/abstract)
 Fraunhofer Institute for Molecular Biology and Applied Ecology IME
Fraunhofer Institute for Molecular Biology and Applied Ecology IME