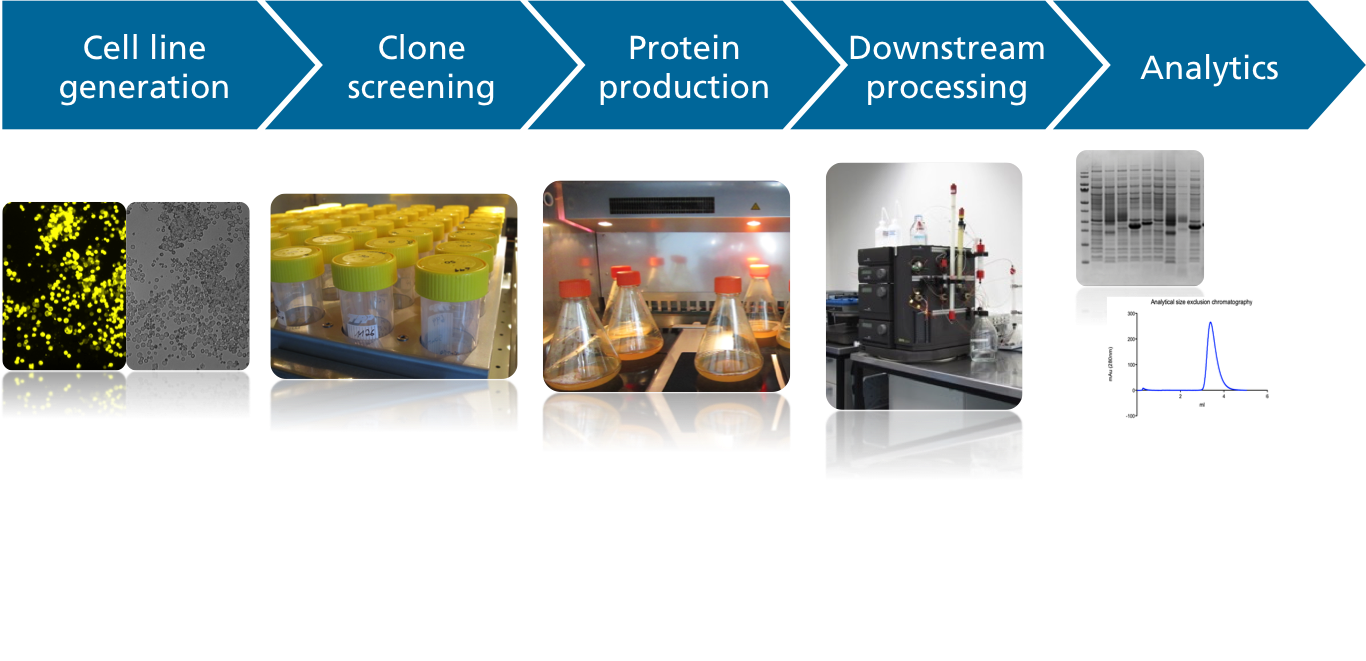
Mammalian cells are excellent hosts for the production of complex recombinant proteins that require extensive folding, the assembly of subunits and/or posttranslational modifications such as N‑glycosylation. For these reasons, mammalian cells are widely used by the pharmaceutical/biotech industry for the production of diagnostic and therapeutic proteins. Different mammalian cell platforms are used according to the quantity and quality of product required.
- Transient expression of recombinant proteins:
Transient expression strategies using HEK293 or CHO cells enable the rapid production of milligram to gram amounts of protein on a flexible scale within a few weeks. The work steps include transfer of the gene of interest into an expression vector, production and purification of the recombinant protein, protein quantification, the determination of protein integrity, and specific functional studies if necessary. - Stable CHO cell line development:
For the development of cell lines that continuously produce a protein of interest, transfected CHO DG44 cells are cultivated under several rounds of methotrexate selection. Monoclonal cell lines are generated by subcloning pools of the most productive cells, and clone stability is confirmed. - Scaled-up production and purification of recombinant proteins (including antibodies):
The scaled-up cultivation of mammalian cells is carried out in stirred-tank bioreactors, single‑use wave-mixed reactors or in single-use orbitally shaken bioreactors at a scale of 1‑100 liters.
 Fraunhofer Institute for Molecular Biology and Applied Ecology IME
Fraunhofer Institute for Molecular Biology and Applied Ecology IME